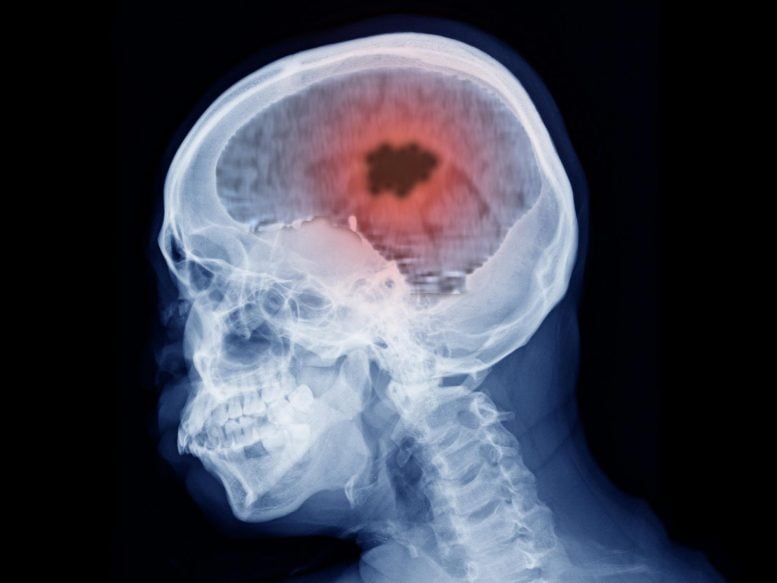
A recent study indicates a significant increase in the risk of developing intracranial meningioma associated with prolonged use of certain progestogens, hormones commonly used in women’s health. This first-of-its-kind study, which utilized data from France’s national health database, highlights the need for more research due to the potential global health implications, given the extensive use of these drugs.
This is the first study to assess the risk associated with widely used progestogens.
Extended use of certain progestogen hormone medications has been linked to a higher risk of intracranial meningioma, a type of brain tumor, according to research from France published recently in The BMJ.
The researchers say this study is the first to assess the risk associated with progestogens used by millions of women worldwide, and further studies are urgently needed to gain a better understanding of this risk.
Progestogens are similar to the natural hormone progesterone, which is widely used for gynecological conditions such as endometriosis and polycystic ovary syndrome, and in menopausal hormone therapy and contraceptives.
Meningiomas are mostly non-cancerous tumors in the layers of tissue (meninges) that cover the brain and spinal cord. Factors such as older age, female sex, and exposure to three high-dose progestogens (nomegestrol, chlormadinone, and cyproterone acetate) are already known to increase the risk of meningioma.
But there are many other progestogens for which the risk of meningioma associated with their use has not been estimated individually.
Study Methodology
To address this knowledge gap, researchers set out to evaluate the real-life risk of intracranial meningioma requiring surgery in women associated with the use of several progestogens with different routes of administration.
They used data from the French national health data system (SNDS) for 18,061 women (average age 58) who underwent intracranial meningioma surgery from 2009-18.
Each case was matched to five control women without intracranial meningioma (total 90,305) by year of birth and area of residence.
The progestogens examined were progesterone, hydroxyprogesterone, dydrogesterone, medrogestone, medroxyprogesterone acetate, promegestone, dienogest, and levonorgestrel intrauterine systems.
For each progestogen, use was defined as at least one prescription in the year before hospital admission or within 3-5 years for levonorgestrel intrauterine systems.
Use of at least one of the three high-dose progestogens known to increase the risk of meningioma in the 3 years before hospital admission was also recorded to minimize bias.
After taking account of other potentially influential factors, prolonged use (a year or more) of medrogestone was associated with a 4.1-fold increased risk of intracranial meningioma requiring surgery. Prolonged use of medroxyprogesterone acetate injection was associated with a 5.6-fold increased risk, and prolonged use of promegestone was linked to a 2.7-fold increased risk.
Observations and Conclusions
There appeared to be no such risk for less than one year of use of these progestogens.
As expected, there was also an excess risk of meningioma for women exposed to chlormadinone acetate, nomegestrol acetate, and cyproterone acetate, all of which are known to increase the risk of meningioma.
However, results showed no excess risk of meningioma for progesterone, dydrogesterone, or the widely used hormonal intrauterine systems, regardless of the dose of levonorgestrel they contained.
No conclusions could be drawn about dienogest or hydroxyprogesterone as the number of exposed individuals was too small.
This is an observational study so can’t establish cause and effect, and the authors acknowledge that the SNDS database lacked information on all the clinical details and medical indications for which progestogens are prescribed. Nor were they able to account for genetic predisposition and exposure to high-dose radiation.
However, they say, given that medroxyprogesterone acetate is estimated to be used for birth control by 74 million women worldwide, the number of attributable meningiomas may be potentially high.
Further studies using other sources of data are urgently needed to gain a better understanding of this risk, they conclude.
Reference: “Use of progestogens and the risk of intracranial meningioma: national case-control study” by Noémie Roland, Anke Neumann, Léa Hoisnard, Lise Duranteau, Sébastien Froelich, Mahmoud Zureik and Alain Weill, 27 March 2024, BMJ.
DOI: 10.1136/bmj-2023-078078








Be the first to comment on "New Research Links Popular Hormone Drugs to Brain Tumors"